A New T(r)ick Up Our Sleeve: Piperacillin as a Novel Treatment for Lyme Disease (Fixations #4)
In our latest post, we discuss a study published this past April identifying a new and more effective drug against Lyme disease.
Introduction
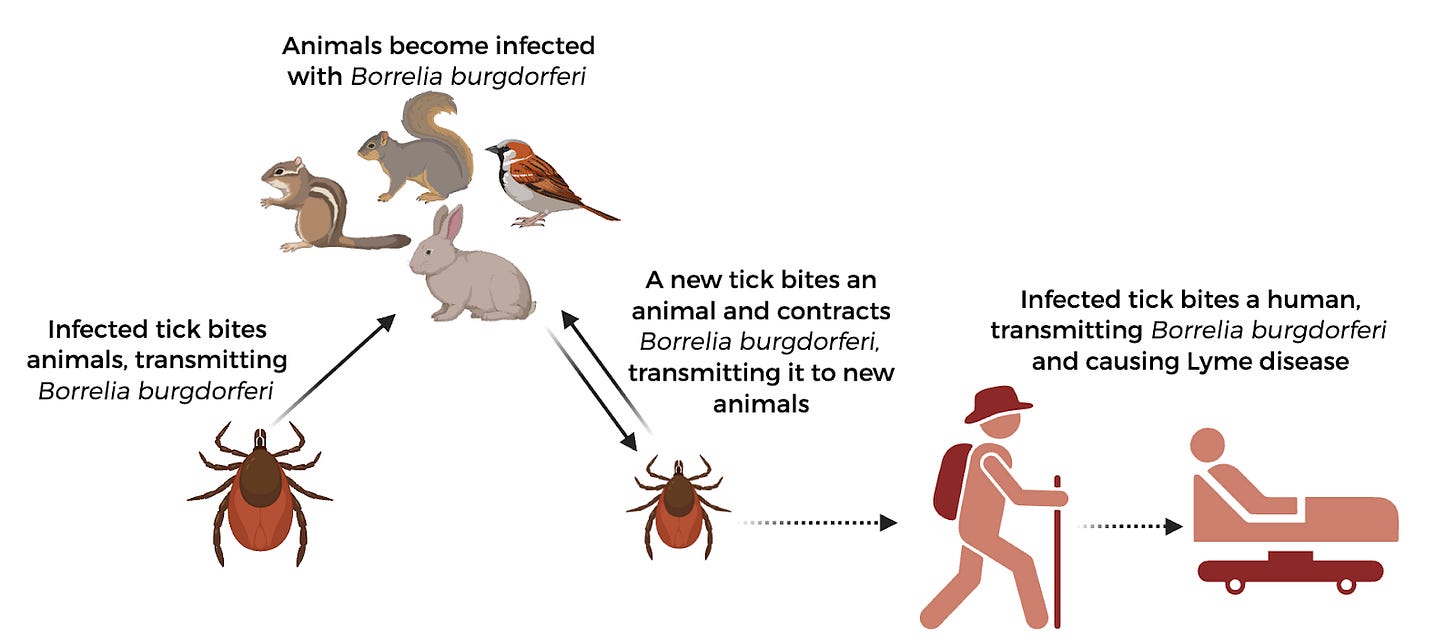
If you’re outdoorsy and love to hike, or if you spend any time in parks, you’re probably familiar with Lyme disease. This bacterial disease is transmitted through the bite of an infected tick–when a tick stays attached to the skin during feeding, the bacteria Borrelia burgdorferi moves through the tick’s saliva into the human, causing an infection. The disease isn’t as uncommon as you think, either; estimates currently place the number of individuals that may be diagnosed with Lyme disease in the United States at a staggering 467,000 each year. Symptoms of infection usually begin with the tell-tale bullseye shaped rash at the site of infection, which is followed by fever and muscle aches. Long-term consequences of this disease include arthritis, difficulty thinking, voice impairment, inflammation of the heart muscles, and body aches that last for months. Importantly, there are currently no vaccines or preventative measures available for Lyme aside from avoiding tick bites.
The current method of treatment relies on a high dosage of an antibiotic called doxycycline, a broad-spectrum drug that not only targets the bacteria that gives rise to Lyme disease (Borrelia burgdorferi), but also damages the body’s native and healthy microbiome. The extended treatment needed to clear the pathogenic bacteria–which is ineffective for about 10-20% of patients–often results in a variety of side effects, such as GI issues or negative impacts on immune function. Additionally, it cannot be administered to young children or pregnant people. Evidently, while we do have an existing treatment for Lyme disease, it may contribute to additional longer-term health issues due to it being a broad-spectrum antibiotic that is not specific to Borrelia burgdorferi.
Thankfully, a group from Virginia Tech and Northwestern University has identified a new antibacterial compound called piperacillin that may serve as a more effective treatment for Lyme. In their study published in Science Translational Medicine this past April, they show that piperacillin specifically targets bacteria from the Borrelia genus by disrupting their unique method of growing their bacterial cell walls. This discovery may pose the solution to the issues of existing methods to treat Lyme disease, which could mean hundreds of thousands of people are closer to a safer way to treat this disease.
Main Findings
Identifying a better drug
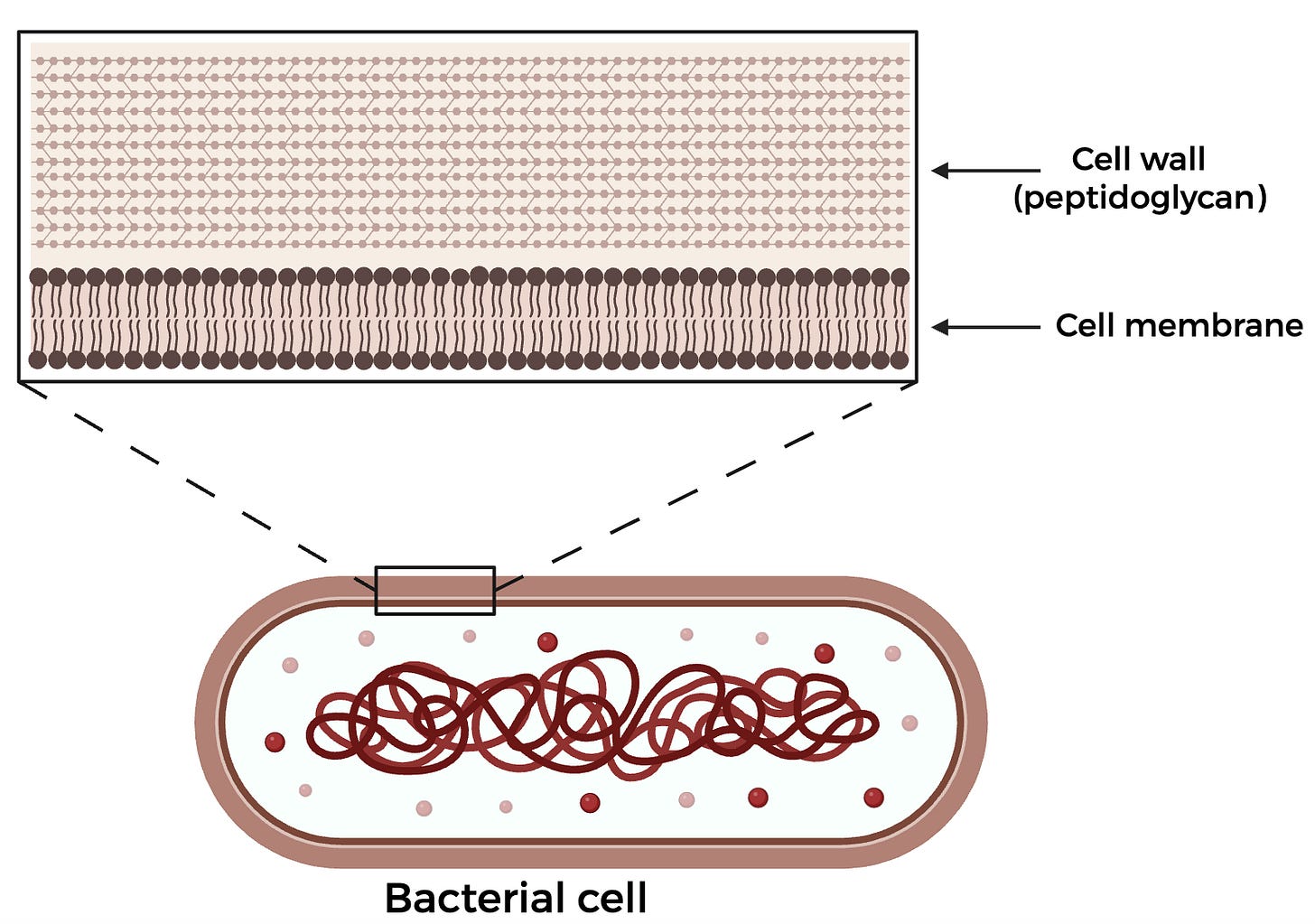
To understand why this drug works, we first must dive into what makes the Lyme disease-causing bacteria B. burgdorferi unique. While your cells have a lipid membrane that surrounds them, bacterial cells have an additional cell wall made of a carbohydrate and protein substance known as peptidoglycan (PG), which forms a thick mesh around the cell. When cells grow and divide, they must build more of this cell wall. Some bacteria with the same morphology–or shape–as B. burgdorferi show PG synthesis across the length of the cell or in one mid-cell zone, corresponding to where the cell will eventually divide. However, B. burgdorferi has been shown to have multiple zones of PG synthesis equally spaced across the cell, making it more difficult to disrupt replication. Since PG synthesis is critical to a bacterial cell’s survival, many commercially available antibiotics–such as penicillin–target this step, which led the authors to hypothesize that some pre-existing FDA-approved antibiotics may be better suited towards specifically inhibiting B. burgdorferi’s mechanism of PG synthesis.
The team began by assembling an initial list of FDA-approved drugs containing 466 compounds, which they subsequently narrowed down to 44 compounds after performing a screen assessing whether the compound was able to restrict growth of B. burgdorferi. Since they were interested in drugs that specifically target B. burgdorferi, they also tested the effect of the drugs against other bacterial strains, including Escherichia coli, Bacillus subtilis, and Staphylococcus aureus. Through these experiments, two major classes of drugs, penicillins and macrolides, showed promising results. After further testing, though, the macrolide drugs were removed from consideration since they have been shown to be less effective in clinical trials. Out of the penicillin class, the compound piperacillin emerged as specific to B. burgdorferi, allowing them to move forward with understanding how the drug works.
How does piperacillin work?
Since the main goal of this study was to identify an alternative for doxycycline, the authors first compared the concentration of piperacillin needed to inhibit growth and kill B. burgdorferi against that of doxycycline. Remarkably, they found that the minimum inhibitory concentration (MIC) for piperacillin was anywhere from 10 to 200 times lower than that of doxycycline, meaning much less of the drug is needed to successfully inhibit bacterial growth. Additionally, they showed that a dose of piperacillin ten times lower than that of doxycycline can significantly reduce the number of bacterial colonies that grow on a plate, indicating that not only is piperacillin more effective at inhibiting growth, it is actually killing the bacteria as well.
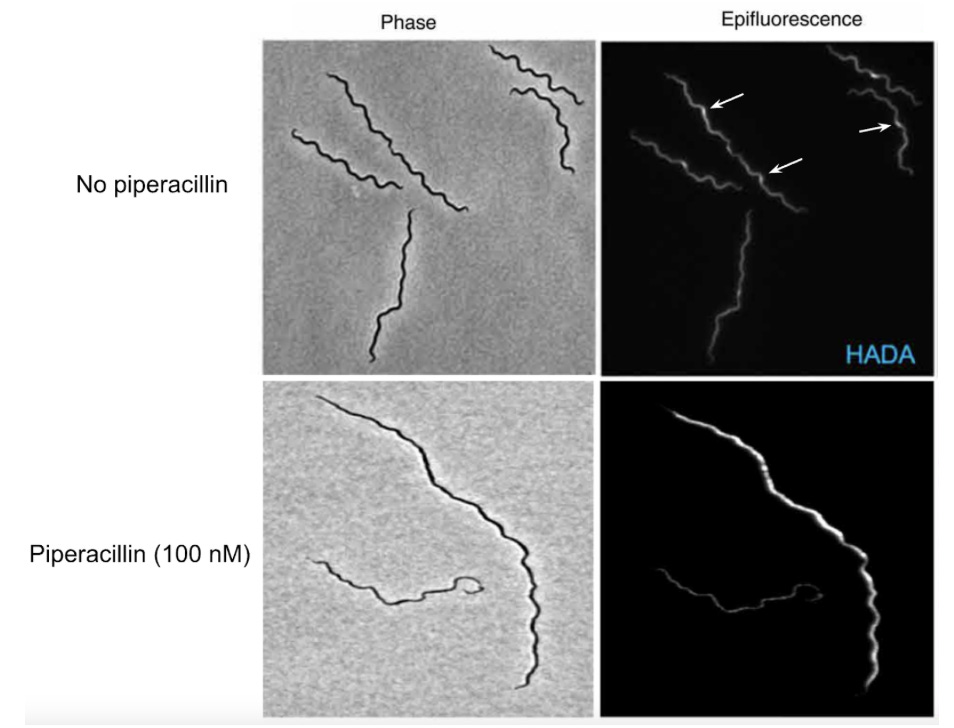
Because piperacillin is part of the penicillin class of drugs–which function by binding to enzymes necessary to build more PG in the cell wall–the authors wanted to better understand how piperacillin was inhibiting PG synthesis in B. burgdorferi. To do this, a fluorescent compound called HADA–which resembles an amino acid used to make PG–was used to monitor cell wall synthesis. If treated with a drug that inhibits PG synthesis, the authors would expect to see less HADA fluorescence when the cells are imaged using a fluorescence microscope. Indeed, cells treated with piperacillin showed an elimination of the PG synthesis zones (Figure 3). Interestingly, the authors also revealed that, following treatment with piperacillin, B. burgdorferi cells had very irregular cell lengths, suggesting that piperacillin’s mechanism of action has to do with cell division. By fusing a fluorescent protein to FtsA–a bacterial cell division protein that helps form a structure called the divisome which allows the cell to divide–they were able to show that, while zones of FtsA were still present following piperacillin treatment, the cells were approximately 1.7 times longer. This indicates that the target of the drug is downstream, or after, the initial formation of the divisome but before cell division.
Knowing now that piperacillin affects PG synthesis and cell division, but not the initial stages of divisome assembly, the group narrowed down their search for piperacillin’s target to a cluster of genes in the B. burgdorferi genome that are involved in PG synthesis. Out of these genes, they found one that encoded for an uncharacterized protein called BB0136, which they hypothesized might be interacting with piperacillin. To test whether BB0136 was being directly bound by piperacillin, they conducted a competition assay, pitting piperacillin against a fluorescent derivative of another drug, penicillin V. By first incubating BB0136 with piperacillin and then adding the fluorescent penicillin V derivative and running the products on an SDS-PAGE gel–used to separate proteins by size–they were able to show that the fluorescence from the penicillin analog shows a rapid decline, indicating that the fluorescent penicillin is being displaced by the piperacillin (Figure 4).
As an additional confirmation, the authors also compared BB0136’s protein structure to other known targets of the drug. Piperacillin has been used to treat Streptococcus pneumoniae, and its target in S. pneumoniae was shown to be a protein called Pbp2X. When the predicted protein structure of BB0136 was overlaid with the S. pneumoniae Pbp2x, the fit was almost perfect, down to the protein’s catalytic active site where the protein carries out its function (Figure 4). This provides further support for their claim that the B. burgdorferi BB0136 protein is the target of piperacillin, as the protein structure aligns with a known target of piperacillin.

Piperacillin in living organisms
When characterizing a new drug, it’s important to understand whether it works as expected in vivo, since many more factors are at play in a living organism than in a controlled test tube environment. This study relied on mice infected with B. burgdorferi, which serve as a useful model to understand drug efficacy in vivo. After infecting mice with the bacteria and allowing the infection to establish itself for 21 days, drugs were administered for one week, including the current Lyme drug doxycycline, piperacillin, and a cocktail of piperacillin and tazobactam–a common cocktail prescribed to patients, as tazobactam inhibits any possible inhibitors of piperacillin. In this first in vivo study, they showed that one week after treatment was finished, the infection was cleared by piperacillin alone with a concentration 100 times lower than that of doxycycline.
In a follow-up in vivo study, the authors were interested in characterizing the timeline of treatment, recovery, and drug dosage, asking whether the mice remained cleared of the infection for several weeks after treatment. At higher doses, piperacillin and doxycycline performed similarly and cleared the infection two weeks post treatment, but another drug ceftriaxone–effective in treating Lyme disease in mice–could not clear the infection at a dose comparable to that of piperacillin. At eight weeks post treatment, no viable bacteria could be recovered from tissues and the joint inflammation associated with infection resolved after only two weeks post treatment. While the authors caveat their results by saying that some bacteria might still be present at low levels in the organs (and could result in a resurgence of infection further out from treatment), they manage to show that B. burgdorferi infection can successfully be cleared for 8 weeks with 100 times less piperacillin than doxycycline.
The major downside to existing doxycycline treatments for Lyme disease is the broad-spectrum antibiotic activity which results in a loss of healthy microbes, particularly in the gut. By assessing the fecal pellets of mice, the authors showed the changes in abundance and diversity of gut microbes in response to treatment with piperacillin, doxycycline, or ceftriaxone. While there was some change in the gut microbiome one day post treatment, the mice treated with piperacillin had much more stable gut microbe diversity than those given the other two drugs. The stability of the microbiome offers added support to their claim that piperacillin is specific to the Lyme disease bacteria, B. burgdorferi, a great added benefit to its efficacy at a significantly lower dose than existing treatments.
Conclusion
While the efficacy of this drug has not been tested against Lyme disease in humans yet, the authors of this study offer a promising potential drug that may provide an improvement to treatment of a significant disease. It is worth noting, however, that the administration method of piperacillin in the mouse studies was not orally, but intraperitoneally–an injection directly into the abdominal cavity to bypass the blood. The intraperitoneal injection method of piperacillin is currently used to treat a variety of severe infections across the body, including the common hospital pathogen Pseudomonas aeruginosa and Streptococcus pneumoniae. Their proposed usage of this drug–following extensive testing–would be a single prophylactic shot in a suspected case of Lyme disease aimed to clear a possible early infection.
While their findings on piperacillin efficacy both in vitro and in vivo are exciting, especially as they pertain to less severe effects on the gut microbiome, there is still a long way to go before we know if the results hold true in humans. The FDA drug approval process can take numerous years, evaluating the efficacy and safety in study participants over 4 phases. Phase 1 pre-clinical trials last about a year and focus largely on safety, side-effects, and pharmacokinetics–how the drug is metabolized and processed in the body. Larger studies understanding the efficacy of the drug against the disease takes place during Phase 2, with a larger scale Phase 3 trial following next and assessing the drug in different types of patients. After approval and marketing, Phase 4 studies investigate long-term usage in a real-world setting, rounding out the process of drug testing and approval. Since piperacillin is already an FDA approved drug, its testing may be started at a later phase, possibly accelerating its marketing as a Lyme drug.
The journey to established therapeutics begins with basic research, with scientists working to unravel the fundamental biology underlying a problem. This work could not have been done without first asking how different bacteria construct their cell walls. From understanding a biological mechanism, to identifying drugs that might help target those mechanisms and studying their mode of action, we owe our life-improving and life-saving drugs to basic research and careful clinical trials. As the last few months have brought attacks on some of our cornerstone research institutions, we must remember that by shutting down or targeting even a handful of institutions, research all over the country will be stalled–or worse, completely halted. Additionally, efforts made to reduce regulatory barriers to manufacturing or testing pharmaceuticals could jeopardize the rigor of controlled clinical trials designed to carefully and adequately test new treatments. The ramifications of these actions will continue to worsen for years, as instability in the scientific world will stall progress in the face of new and continuing diseases. It simply cannot be overstated: these actions will have lasting impacts on our country’s health. With new threats like avian flu on the horizon, we stand at a perilous tipping point. If we want to stand a chance against life-threatening diseases–both those we know and those we don’t–it is critical to protect our nation’s research institutions and push back on cuts to research funding. Now, before it’s too late.