By The Skin of Your... Exoskeleton? The Evolutionary Origins of Modern Teeth (Fixations #5)
In our latest Fixations post, we explore how modern teeth may have evolved from sensory tissues in the exoskeletons of early vertebrates.
Introduction
When you think about your teeth, you might conjure up the fear of going to the dentist or memories of growing up with braces, but do you conjure up the hard exoskeletons of ancient fish? Turns out, you should be. In a recent study published by researchers at the University of Chicago and Harvard’s Museum of Comparative Zoology, the authors reveal that dentine–mineralized tissue in your teeth that is crucial for sensory function–actually evolved as a sensory tissue in the exoskeleton of early vertebrates. But first, let’s back-track: why is this significant, and why were the authors attempting to pinpoint the evolutionary origins of dental tissues like dentine?
To answer these questions, we need to first review what we know about teeth and how they evolved. Teeth didn’t start out in the form we know them now, nor did they start in the mouth; modern teeth evolved from odontodes, or structures located in the dermal exoskeleton of jawless vertebrates. In other words, before jaws had evolved, “teeth” structures were located on the surface of the skin. These odontodes were made of dentine (the tissue we discussed earlier) and originated from a cell population only found in vertebrates called neural crest cells. While we’ve been able to chronicle and characterize the evolutionary journey of odontodes to modern-day teeth and other structures, however, where these structures themselves came from is still unclear.

At the time of this study, scientific consensus appeared to point to Anatolepis heintzi, a fossil considered the first fish, as the first example of odontodes. While there had been some controversy over whether the fossil really represented a vertebrate or not, a subsequent study showing the composition of its odontode-specific tissues restored it to its position as the first fish. While this back-and-forth exchange may look like mere scientific quibbling to us, knowing this position matters because it has significant implications for not only when tissues like dentine evolved, but also for why odontodes evolved in the first place. In the study we’ll be discussing in this piece, the authors sought out to analyze the origins and distribution of dentine by looking at the exoskeletons of both fossils and existing vertebrate and invertebrate organisms; in doing so, their results shed light on the evolutionary trajectory of odontodes and raise new possibilities regarding their function in early vertebrates.
Main Findings
To kick off their study, the authors decided to revisit the question of Anatolepis heintzi’s vertebrate status using a technique called high resolution synchrotron tomography. As we’ve discussed in the past, CT-based techniques allow researchers to analyze the internal features and tissues of their sample of interest. Usually, X-rays are shot at the sample from all angles to build a 3D representation of the sample, which can be segmented and analyzed computationally. In this case, however, the authors used Argonne National Laboratory’s particle accelerator to scan their samples in order to achieve the resolution necessary to assess the structure and compositions of their tissue of interest in Anatolepis. Importantly, this method is non-destructive, a necessity when you’re working with delicate, fossilized samples.
Using this technique, the authors analyzed fossils of Anatolepis as well as those of extinct marine arthropods (think ancient crabs) to assess whether Anatolepis odontodes truly resemble those of vertebrates. Surprisingly, they found that this wasn’t the case. Scans revealed that both fossils bore the same structural hallmarks in their respective cuticles (think tough, protective parts of the skin), as shown in the figure below: vertical and horizontal canals, a central cavity (seen in purple, denoted by cc), cuticular organs with a teardrop shape (seen in yellow, denoted by co), and tubules with an arrowhead shape (indicated by asterisks, denoted by tb). These anatomical similarities led the authors to conclude that Anatolepis has, in fact, been misidentified as a vertebrate and bears more similarity to marine arthropods.

To follow up on this surprising finding, the authors decided to analyze the exoskeletons of both vertebrate and invertebrate samples from fossils and present-day organisms in order to assess their overall similarities. Here, they found that the cuticles of Anatolepis and their marine arthropod comparison did not resemble the dental tissues or bone seen in the vertebrate samples, but instead resembled the sensory organs of the modern arthropods they analyzed. Of these, the authors decided to hone in on a crustacean called the porcelain crab and the microanatomy of its sensilla, or a sensory organ that might play a role in feeding. They saw that, despite some differences, such as how the tubules were arranged (all on one side in the crab compared to circumferentially in Anatolepis & co.), the structures bore significant resemblance to each other. This is important for two reasons: not only does it serve as further confirmation of Anatolepis’s invertebrate status, it also reveals something about its cuticles–they may have had a sensory function.
Before they got too far into the weeds of invertebrate sensory structures, however, the authors decided to examine the odontodes and dentine of known vertebrate fossils dating to the Middle Ordovician (between 470 and 458 million years ago) and compare them to the invertebrate structures. More specifically, they analyzed fossils from the two most studied taxa of the period, Eriptychius and Astraspis, which you can think of as ancient jawless fish (and, in the case of Eriptychius, the oldest known evidence for the development of the vertebrate head!). Focusing on the former of these, the authors noted that the fossil’s odontodes have a distinct morphology, are made up entirely of dentine, and lack a capping enameloid structure.
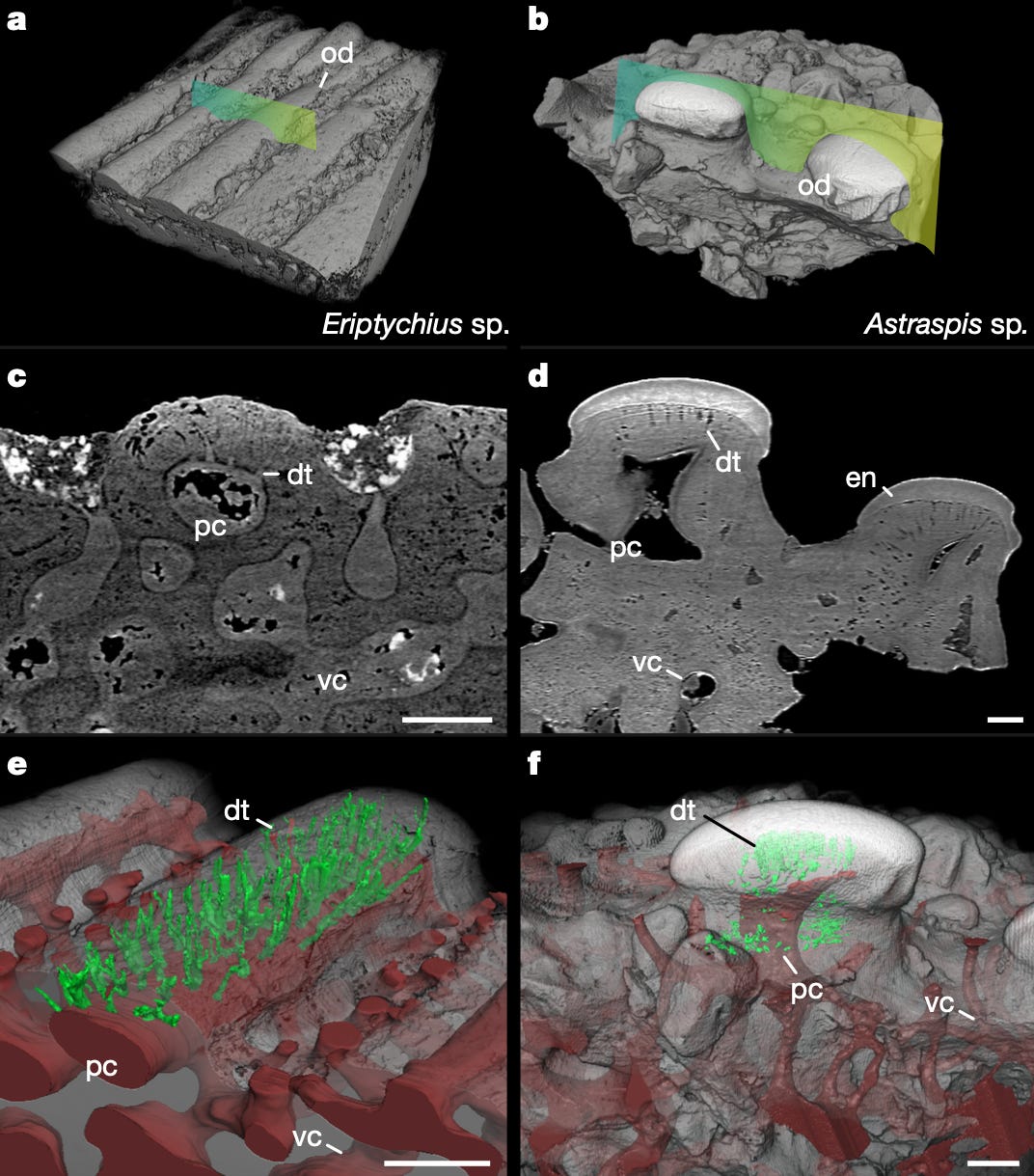
This last detail is important–without an enamel cap, the dentine in the odontodes would be exposed to the environment. Given that dentine is known to be important for transmitting environmental information to the nerves, this raises the possibility that odontodes were involved in some sensory function, like sensing temperature or pressure in the water. This is supported by other attributes identified by the authors–namely, the presence of an open cavity (indicated by pc in the figure below) in the structure and the tubules’ connection (indicated by dt) to the surface of the skin–which are indicative of integration with nerves and vasculature and overall involvement with sensory function, respectively. Interestingly, the authors observed that tubule connection to the skin was seen in both the marine arthropods and Eriptychius, suggesting that both vertebrate and invertebrate organisms evolved a similar mechanism for sensing the external environment–albeit one that varies slightly in composition and structure.
To round out their study, the authors were interested in understanding whether this ancestral sensory function persists in the odontodes of present-day organisms by analyzing the external odontodes of a variety of fish. Because these structures are homologous to our oral teeth, the authors assumed that they would also be innervated, or connected to nerves. To test their hypothesis, they used immunofluorescence (using fluorescent antibodies to label something of interest, like a protein) and confocal microscopy to label nerves in their samples of choice–juvenile catsharks, little skates, and a bristlenose catfish–and see if their odontodes were innervated. Indeed, this is what they saw in every organism assayed; in the chondrichthyans (cartilaginous fish), they observed nerves at the base of the odontode, while the catfish had nerves invading the pulp cavity of the odontodes in its pectoral fin. This commonality suggests that the association of nerves with dentine is an ancestral trait, meaning that it is a characteristic inherited from the ancestors of these organisms.

Taken together, this study paints a fascinating picture of how odontodes may have functioned in early vertebrates and how our knowledge about these exoskeletal features can be connected to our modern oral teeth. Using high-resolution CT scanning techniques, the authors reveal that the cuticles of what was thought to be the first fish, Anatolepis, actually resemble those of a group of marine arthropods called aglaspidids (which themselves resemble sensory organs of modern invertebrate species like the porcelain crab). When they looked at vertebrate fossils, and particularly those of the taxa Eriptychius, the authors found that their odontodes were made up of dentine and exposed to the environment, suggesting an ancestral sensory function–one that, for all intents and purposes, continues in present-day odontodes structures and likely represents the early antecedents to our wonderfully sensitive oral teeth.
Takeaways
While there is so much to take away from this study–how vertebrates and invertebrates developed similar, but independent, methods of sensing their environment, how odontodes set the stage for our teeth, or how there is still so much to learn from our fishy ancestors–what drew me to this particular work was actually the story behind it. In an interview with NPR, lead author and evolutionary biologist Dr. Yara Haridy talks about how this study had a very different start; initially, she had set out to understand the evolution of our skeletons, with Anatolepis as her main subject of analysis. As Haridy tells it, the finding that Anatolepis wasn’t a vertebrate “signified that my project was almost broken.”
After discussing the finding with her advisor, Dr. Neil Shubin, however, Haridy and her colleagues turned to the question of teeth, giving rise to the study we’ve discussed here today. And so, a project that was initially about understanding where our bones came from became a study that revealed the evolutionary origins of teeth as sensory structures. While scientists are no strangers to the idea of a new result or data point turning a hypothesis or project on its head, we don’t often appreciate just how often this happens and how simple curiosity can lead us to discover things we hadn’t even set out to look for.
Because of the way we learn and treat scientific facts as established or sometimes even immutable truths, it can be easy to forget that not everything is as set in stone as we assume it to be. Another reason I love this study is because it simultaneously reminds us that we don’t necessarily know everything we think we know, and there is still so much left to learn. Additionally, as technology continues to evolve (ha, see what I did there?), we can revisit subjects like Anatolepis and see if there’s a new way of understanding or characterizing something, either through a new lens or a new method. Ultimately, while we are part of an era in biology filled with unprecedented advanced methods of generating and acquiring data, there is much left to learn–and we should never forget or underestimate that.
So, the next time you’re on the unfortunate end of a dental tool or suffering through a toothache brought on by one too many sweets, or questioning a data point that doesn’t make sense under your current hypothesis, think of these ancient jawless fish and of all the things we have left to learn from them and the world they once inhabited.